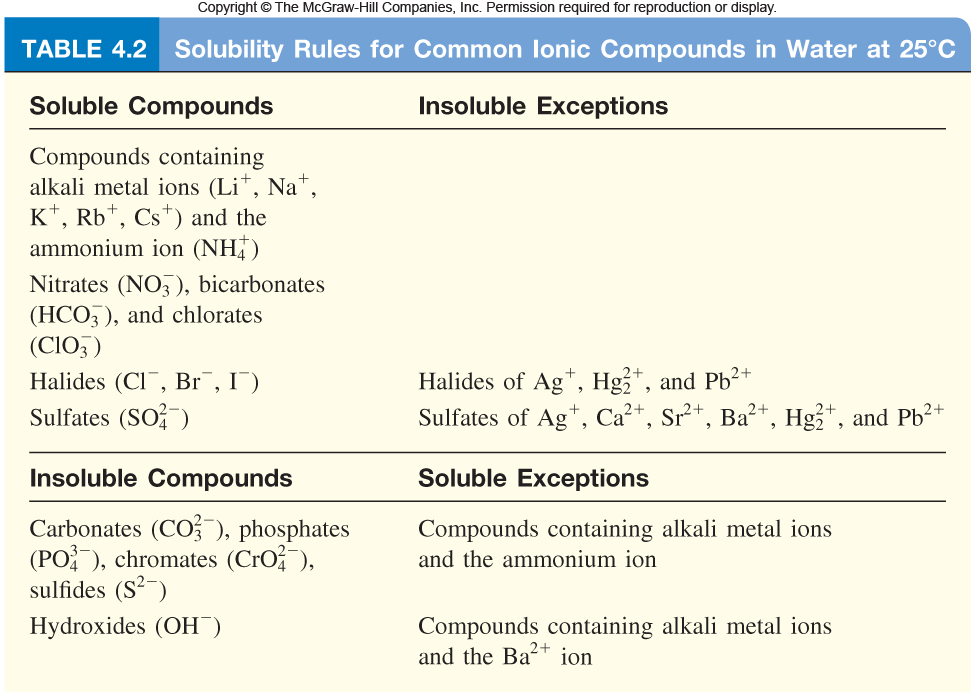
What Ionic Compounds Are Insoluble In Water . when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. this is a list of the solubility rules for ionic solids in water. Solubility rules can be used to predict whether a large number of ionic. Solubility is a result of an interaction between polar water molecules and the. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. not all ionic compounds are soluble in water.
from dxonxarcb.blob.core.windows.net
solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Solubility is a result of an interaction between polar water molecules and the. review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Solubility rules can be used to predict whether a large number of ionic. not all ionic compounds are soluble in water. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. this is a list of the solubility rules for ionic solids in water.
What Type Of Substances Will Dissolve In Water To Form Aqueous Solutions at Lois Witherspoon blog
What Ionic Compounds Are Insoluble In Water Solubility is a result of an interaction between polar water molecules and the. not all ionic compounds are soluble in water. Solubility rules can be used to predict whether a large number of ionic. this is a list of the solubility rules for ionic solids in water. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. Solubility is a result of an interaction between polar water molecules and the.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID1926480 What Ionic Compounds Are Insoluble In Water this is a list of the solubility rules for ionic solids in water. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic. What Ionic Compounds Are Insoluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 What Ionic Compounds Are Insoluble In Water ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. Solubility is a result of an interaction between polar water molecules and the. this is a list of the solubility rules for ionic solids in water. not all ionic compounds are. What Ionic Compounds Are Insoluble In Water.
From www.transtutors.com
(Solved) Some Solubility Rules For Ionic Compounds In Water Are Shown For... (1 Answer What Ionic Compounds Are Insoluble In Water Solubility rules can be used to predict whether a large number of ionic. review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given. What Ionic Compounds Are Insoluble In Water.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is completely dissolved into aqueous What Ionic Compounds Are Insoluble In Water review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Solubility rules can be used to predict. What Ionic Compounds Are Insoluble In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Solutions at Lois Witherspoon blog What Ionic Compounds Are Insoluble In Water this is a list of the solubility rules for ionic solids in water. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Solubility is a result of an interaction between polar water molecules and the. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. What Ionic Compounds Are Insoluble In Water.
From www.numerade.com
SOLVED Question 9 (1 point) Compounds in Water at Room Temperature Indicate the solubility What Ionic Compounds Are Insoluble In Water review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic.. What Ionic Compounds Are Insoluble In Water.
From www.slideserve.com
PPT Solubility Rules & Net Ionic Equations PowerPoint Presentation ID2740106 What Ionic Compounds Are Insoluble In Water this is a list of the solubility rules for ionic solids in water. not all ionic compounds are soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. . What Ionic Compounds Are Insoluble In Water.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions PowerPoint Presentation ID What Ionic Compounds Are Insoluble In Water Solubility rules can be used to predict whether a large number of ionic. review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. this is a list of the solubility rules for ionic solids in water. not all ionic compounds are soluble in water. Solubility is a result of an interaction. What Ionic Compounds Are Insoluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 What Ionic Compounds Are Insoluble In Water not all ionic compounds are soluble in water. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Solubility rules can be used to predict whether a large number of ionic. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy. What Ionic Compounds Are Insoluble In Water.
From chemcollective.org
CHEM 1315 Lab 10 Conservation of Mass What Ionic Compounds Are Insoluble In Water this is a list of the solubility rules for ionic solids in water. Solubility rules can be used to predict whether a large number of ionic. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. What Ionic Compounds Are Insoluble In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Solutions at Lois Witherspoon blog What Ionic Compounds Are Insoluble In Water not all ionic compounds are soluble in water. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. this is a list of the solubility rules for ionic solids in water. Solubility is a result of an interaction between polar water molecules and the. ionic compounds dissolve in water if the. What Ionic Compounds Are Insoluble In Water.
From chem1180.blogspot.com
CHEM 1180 17.1 The Solubility Product Constant What Ionic Compounds Are Insoluble In Water review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. this is a list of the solubility rules for ionic solids in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. solubility is the maximum amount of solute that. What Ionic Compounds Are Insoluble In Water.
From www.chegg.com
Solved Categorize each of the following ionic compounds as What Ionic Compounds Are Insoluble In Water solubility is the maximum amount of solute that can dissolve in specific amount of solvent. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. this is a list of the solubility rules for ionic solids in water. Solubility rules can. What Ionic Compounds Are Insoluble In Water.
From www.chegg.com
Solved Part A Predict whether each of the following ionic What Ionic Compounds Are Insoluble In Water Solubility is a result of an interaction between polar water molecules and the. Solubility rules can be used to predict whether a large number of ionic. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. not all ionic compounds are soluble in water. this is a list. What Ionic Compounds Are Insoluble In Water.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Visionlearning What Ionic Compounds Are Insoluble In Water review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. Solubility rules can be used to predict whether a large number of ionic. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. solubility is. What Ionic Compounds Are Insoluble In Water.
From www.chegg.com
Solved Which of the following ionic compounds is insoluble What Ionic Compounds Are Insoluble In Water review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. not all ionic compounds are soluble in water. Solubility is a result of an interaction between polar water molecules and the. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the. What Ionic Compounds Are Insoluble In Water.
From www.slideserve.com
PPT CHE 111 Module 4 PowerPoint Presentation, free download ID694735 What Ionic Compounds Are Insoluble In Water solubility is the maximum amount of solute that can dissolve in specific amount of solvent. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium.. What Ionic Compounds Are Insoluble In Water.
From ar.inspiredpencil.com
Ionic Compounds Chart What Ionic Compounds Are Insoluble In Water Solubility is a result of an interaction between polar water molecules and the. not all ionic compounds are soluble in water. this is a list of the solubility rules for ionic solids in water. solubility is the maximum amount of solute that can dissolve in specific amount of solvent. ionic compounds dissolve in water if the. What Ionic Compounds Are Insoluble In Water.